Biomedical reliability of electronics
- Home
- >
- Research
- >
- Biomedical reliability of electronics
Malfunctions of biomedical devices are extremely critical in patient diagnosis and treatment, and thus the reliability of bioelectronics play an important role during the translational and commercialization stage of newly developed techniques. Biofluid, a mixture of various elements induces electrochemical reactions and the movement and metabolism of organ systems cause mechanical degradation. Biological immune reactions to foreign bodies must be considered for realization of medical tools. This topic covers understanding and evaluating the various factors that determine the lifetime and stability of implanted devices and providing methodologies to ameliorate reliability problems.
Degradation mechanisms of medical devices in biofluids
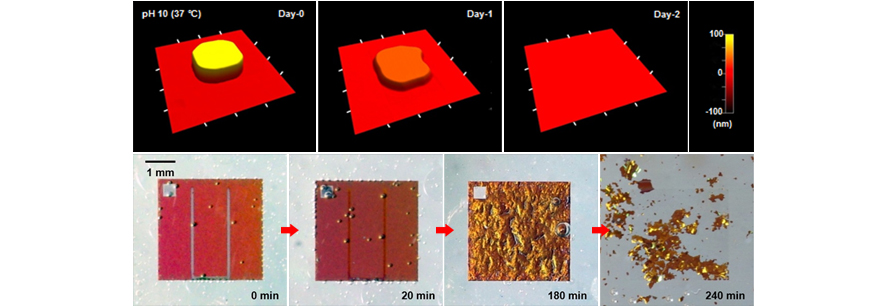
Biofluid, a mixture of various kinds of ions, molecules and enzymes, can degrade implanted devices. Evaluation of degradation mechanisms in biofluid makes it possible to predict device lifetime in the body. Below are images of degradation of Si nanomembrane measured by atomic force microscopy (AFM) associated with consistent dissolution by oxidation.
Biocompatibility of materials and devices
Foreign materials implanted in the body cause foreign-body reactions and accompanying immunochemical reaction and infection. Biocompatibility testing is thus a primary concern in applying biomedical systems. There are several stages and levels of evaluation of biocompatibility: the cell live/dead test and proliferation test two such evaluations, and an advanced example is implantation in a living animal to observe the immune reaction and histological information of tissue. The example below shows histological brain tissue near dissolved pressure sensors made up of Si, Mg, and SiO2.

Evaluation of mechanical reliability
Mechanical degradation dominates the reliability problem in biomedical electronics. Our biological systems are always moving and our biofluids are circulating. These repeating and/or complex forces shorten the lifetime of electronic devices. Thus, evaluating the mechanical lifetime of biodevices is important to provide guidelines on stable device operation. Nanoindentation is one of the techniques for evaluating the mechanical properties of thin-film devices.

References
- S.-K. Kang et al. "Dissolution chemistry and biocompatibility of Si- and Ge-based semiconductors for transient electronics",
ACS Applied Materials & Interfaces 7, 9297-9305 (2015). - S.-W. Hwang et al. "Biodegradable Elastomers and Silicon Nanomembranes/Nanoribbons for Stretchable, Transient Electronics and Biosensors", Nano Letter 15, 2801 (2015).
- S.-K. Kang et al. "Biodegradable Thin Metal Foils and Spin-On Glass Materials for Transient Electronics",
Advanced Functional Materials 25, 1789 (2015). - S.-K. Kang et al. "Dissolution behaviors and applications of silicon oxides and nitrides in transient electronics",
Advanced Functional Materials 24, 4427 (2014). - X. Huang et al. "Biodegradable Materials for Multilayer Transient Printed Circuit Boards",
Advanced Materials 26, 7376 (2014). - S.-W. Hwang et al. "Dissolution Chemistry and Biocompatibility of Single-Crystalline SiliconNanomembranes and Associated Materials for Transient Electronics", ACS Nano, 8, 5843 (2014)
- L. Yin et al. "Dissolvable Metals for Transient Electronics", Advanced Functional Materials 24, 645 (2014).
- S.-K. Kang et al. "Extended expanding cavity model for measurement of flow properties using instrumented spherical indentation", International Journal of Plasticity 49, 1 (2013).
- S.-K. Kang et al. "Determining effective radius and frame compliance in spherical nanoindentation using an infinitesimal deformation concept", Materials Science Engineering A 538 (2012) 58.
- G. Lee et al. "Characterization of elastic modulus and work of adhesion in elastomeric polymers using microinstrumented indentation technique", Materials Science Engineering A 496, 494 (2008).

